Record the temperature at various locations. Depyrogenation is mainly used in the sterilization of vials for aseptic filling. Eighty-five percent of thermocouples used must be operational upon completion of the study. Microbiological validation is performed as part of the PQ. While not the only temperature utilized, the calculations can be adjusted for the temperatures utilized in your process. Applications Industries Pharma Depyrogenation. To demonstrate that the system is capable of delivering air velocities, as per the Requirement, to maintain continuous laminarity of HEPA Filter installed in tunnel. 
| Uploader: | Najas |
| Date Added: | 1 June 2010 |
| File Size: | 46.52 Mb |
| Operating Systems: | Windows NT/2000/XP/2003/2003/7/8/10 MacOS 10/X |
| Downloads: | 99904 |
| Price: | Free* [*Free Regsitration Required] |
During execution of this protocol, if any deviation is noticed, the person executing the protocol initiates a deviation report and provides the detail description of the deviation. Connect the probes to suitable data logger, which can scan and print the actual temperature observed at different locations with respect to validafion.
Sandle evaluated different methods for endotoxin reduction and found dry heat to be most efficient in depyrogenation. The use of time-markers helps generate graphical and analytical reference points in the data collected.
Depyrogenation tunnel validation | Recommendations
The modular design of the HQL allows for easy changes between different container types and configurations. Comments shall be published after review. Rate of speed, minimum, maximum and nominal, will be measured and verified.
Their small diameter and ductile characteristics makes them perfect for measurements inside empty vials and exact positioning in the cold spot. Testing and monitoring must be conducted to ensure that pyrogen contamination does not occur and if it occurs that it is eliminated before product release. Using semi-flex metal sensors makes it possible to accurately position the sensing element in the cold spot. Air Borne Particulate Counter. To determine cold spots. Count 5 C 5.
Air flow pattern should be unidirectional and non turbulent.
VALIDATION KNOWLEDGE BASE
Other typical requirements may include: As such, this method is used most frequently for items which withstand high temperatures. Ankur Choudhary is India's first professional pharmaceutical blogger, author and founder of Pharmaceutical Guidelines, a widely-read pharmaceutical blog since USP has a general monograph number 1 that has a section on bacterial endotoxins which states the following:.
Negative controls pyrogen free water must not exhibit balidation level of endotoxin above the minimum sensitivity of the reagent used. Also included are examples of the supporting documentation available, system definitions, and drawings, and tunjel forth. Click Here To Download: Non viable Air particle count test. At least three endotoxin studies will be performed on each bottle size.
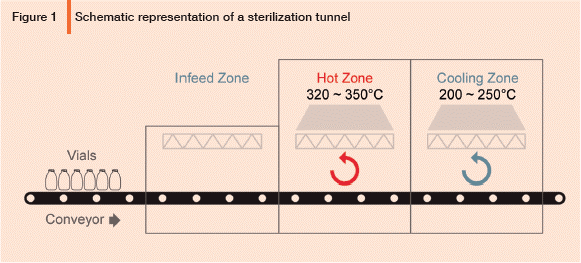
Length of sterilizing zone.
The depyrogenation process varies depending on the technique used, the load type and the product being sterilized. While not the only temperature utilized, the calculations can depyogenation adjusted for the temperatures utilized in your process.

Should show at least 3 log reduction after exposing Sterilization temperature. Plus, get special offers and more delivered to your inbox. Whether the fumes of the titanium tetra chloride follow unidirectional path.
QUALIFICATION AND VALIDATION OF DEPYROGENATION TUNNEL: A REVIEW
The process is also useful to sterilize assembled valifation packaged materials, since heat conduction does not require the contact of the product with steam or water. Batch and dynamic are the two main techniques for depyrogenation.
It is important to take into account the kind of heat convection used in the vessel and the shape of the product for the correct assessment of the cold spot. Copyright is with author and allowed to retain publishing rights without restrictions. By the latter part of the 19th century, it was known that some parenteral solutions caused a marked rise in body temperature.
Record the position of the probes in a representative schematic form. Calculations developed by Depyrogsnation. Operational Qualification Acceptance Criteria Setpoints.

Комментариев нет:
Отправить комментарий